How Chronic Infections May Drive Neurological Disease
A break down of several studies drawing links between neurological conditions and prior infections
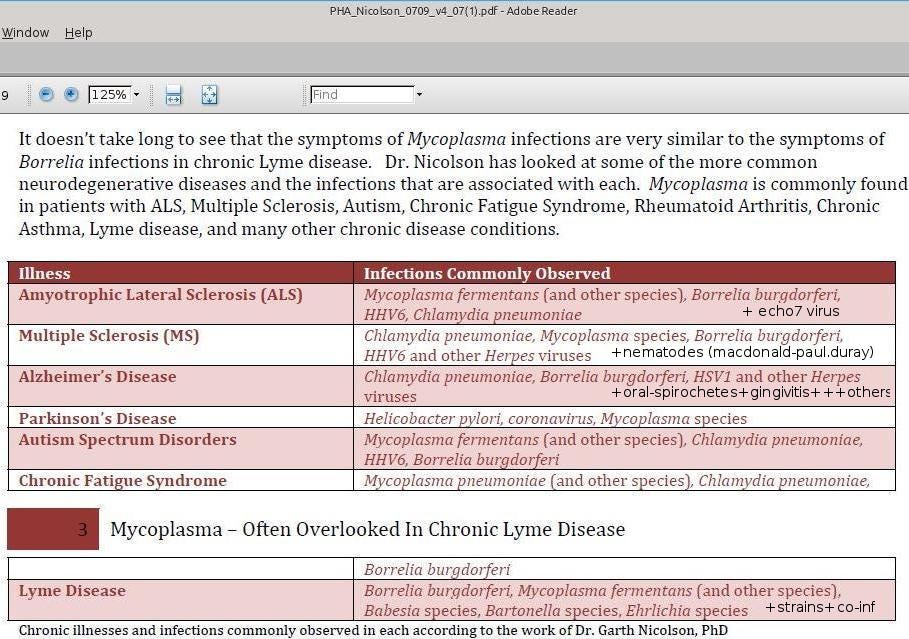
Neuro-infections occur when infectious agents such as bacteria, viruses, or parasites invade the brain or spinal cord, leading to inflammation, immune disruption, and long-term neurological damage. While acute conditions like meningitis are well known, a growing body of research suggests that chronic or latent infections may silently contribute to a range of degenerative disorders once thought to be purely genetic or autoimmune.
One group of microbes called spirochetes have received particular attention in the following research papers. These bacteria are capable of invading the brain and establishing persistent infections. They can spread not only through the bloodstream and lymphatic system, but also along nerve pathways. Oral spirochetes, for example, have been found in the trigeminal nerve and ganglia, and may even reach the brain via the olfactory system: one of the first regions affected in Alzheimer’s disease.
These studies and reviews trace the evolving understanding of the subject over two decades. Each section reflects the scientific findings and discourse available at the time of publication, and may not present the current paradigm.
Disease Summary, Symptoms and Diagnosis
From a narrative review in 1989, by Andrew R. Pachner: Lyme disease, caused by a tick-borne spirochete bacteria called Borrelia burgdorferi, can affect the nervous system in ways that closely resemble other serious conditions: multiple sclerosis, brain tumors, psychiatric disorders. Pachner repeatedly uses syphilis as a comparison due to the commonalities in symptoms between the two infections. Lyme disease can be helpfully understood in three stages, though it presents differently depending on each patient. It should be noted that there can be latent periods between symptomatic stages.
Stage One – Early Localized Infection
Occurs days to weeks after tick bite
May involve a skin rash called erythema chronicum migrans (ECM) and viral-like systemic symptoms such as fever, fatigue, and headache
Likely that some of the organism disseminates to the central nervous system (CNS) during this stage
Stage Two – Early Disseminated Infection
Develops a few weeks to a few months after the initial infection
Common signs include Lyme meningitis (often combined with facial palsy), cranial neuropathies, and polyneuritis
Stage Three – Late Disseminated Infection
The third stage involves parenchymal brain involvement.
It is confirmed that Borrelia burgdorferi can invade brain tissue, but the frequency of this invasion compared to cerebrospinal fluid (CSF) or leptomeningeal involvement is uncertain
The Lyme bacterium appears to be highly neurotropic (tending to affect nervous tissue) and may remain dormant in the brain for years before triggering neurological symptoms. Diagnosis tends to be difficult since symptoms are nonspecific and may appear long after the initial infection. Competent doctors rely on high antibody levels, spinal fluid abnormalities, and treatment response to confirm cases. Many overlook Lyme diagnosis when there is no history of a skin rash, though this is a flawed approach.
Common misdiagnosis include:
Aseptic meningitis, rather than bacterial meningitis.
Bell’s palsy which will resolve with time
Hysteria, related to the neurologic manifestations of stage two
Due to the complexity of the disease and frequent failure to identify it, there is an insufficient understanding of how it functions. Many questions remain about its behaviour in the brain, how long it can stay dormant, and how best to treat advanced neurologic Lyme disease. Pachner presents an outline on how to make a diagnosis (all five must be present to be definitive)
Diagnostic criteria for stage three:
Objective CNS involvement, such as neurologic signs on physical exam, MRI findings, or clear psychiatric manifestations
Reproducible, high-titer Lyme serology, excluding low-titer results to avoid false positives
Evidence of other organ involvement, such as erythema chronicum migrans (ECM), arthritis with inflammation, or meningitis
Lymphocytic pleocytosis in the cerebrospinal fluid (CSF) Requires spinal Tap
Positive clinical response to intravenous (injected into veins) antibiotics, supporting the diagnosis retrospectively
A Likely Connection Between ALS and Lyme Disease
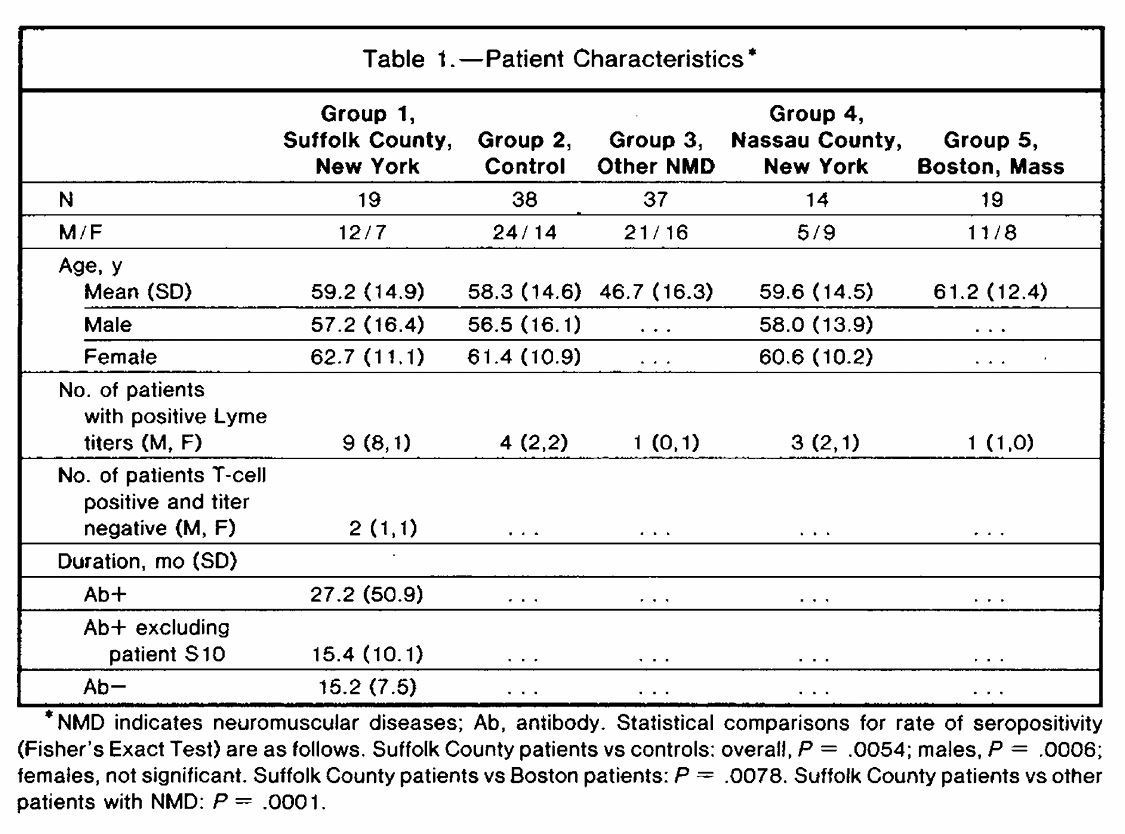
A 1990 study published in the Archives of Neurology identified a compelling association between Lyme disease exposure and Amyotrophic lateral sclerosis (ALS) in a Lyme-endemic region of New York.
Lyme borreliosis often leads to peripheral neuropathies and radiculopathies, with symptoms sometimes persisting for months to years if left untreated. Meanwhile, ALS is a more uncommon, yet untreatable neurological issue.
Among 19 unselected ALS patients from Suffolk County, 9 (47%) tested positive for Lyme disease antibodies using ELISA, compared to only 4 of 38 matched healthy controls (10.5%). The disparity was especially pronounced among men: 67% of male ALS patients were seropositive, compared to just 8% of male controls. This difference was statistically significant (p = 0.0006), indicating a mere 0.06% probability that the association between Lyme exposure and ALS occurred by chance in this sample.
None of the ALS patients in the study showed classic signs of Lyme disease, such as rash, joint pain, periodontal disease, or a known history of tick bite. Despite living in a region with high Lyme prevalence, their medical histories and symptoms gave no indication of prior Lyme infection. However, a small number of patients—particularly those in the early stages of ALS with predominantly lower motor neuron involvement—showed unexpected clinical improvement or stabilization after a two-week course of intravenous antibiotics. The treatment did not affect bulbar abnormalities. The partial success of this treatment is highly unusual, given that ALS is a disease that typically progresses without remission.
Patients with more advanced illness often deteriorated rapidly or died shortly after antibiotic treatment. The authors suggested this could reflect a Herxheimer-like reaction. This is a reaction which is uncomfortable but usually harmless, reflecting a temporary immune response to bacterial breakdown. However, in these severely affected patients, the resulting inflammation may have posed a greater risk than usual. More broadly, the authors proposed that Borrelia burgdorferi infection may occasionally cause a motor polyradiculoneuritis, a condition affecting the ventral nerve roots, which could mimic ALS in its early stages.
The differing outcomes in patients indicate that timing, disease severity, and the pattern of motor neuron involvement are critical in determining whether antibiotic therapy offers a positive risk-to-benefit ratio in ALS cases linked to unrecognized Lyme exposure.
In summary, this study introduces the possibility that a treatable infectious trigger may underlie a subset of ALS-like syndromes in Lyme-endemic regions. While causation remains unproven, the evidence supports more rigorous screening for Lyme disease in early ALS cases, especially those with lower motor neuron signs. Early treatment may alter the course of illness for a small but significant group of patients once thought untreatable.
Lyme Disease Brain Inflammation
A 1996 study examined three patients with serious brain inflammation caused by Lyme disease affecting the nervous system, known as Lyme neuroborreliosis (LNB). Their symptoms included seizures, memory problems, walking difficulties, and partial paralysis. Brain scans (MRI) showed abnormal white matter lesions. In two patients, the lesions were mistaken for brain tumors until further testing confirmed LNB. This confusion is common because LNB can look like many other diseases.
Possible Misdiagnoses:
Neurosyphilis
Viral, fungal, or bacterial meningoencephalitis
Multiple sclerosis
Brain tumor
Autoimmune disease
Stroke
Alzheimer's disease
All three patients had inflammation of blood vessels in the brain (vasculitis). One patient, who’s condition was fatal, showed large areas of brain tissue damage called demyelination (similar to what is seen in multiple sclerosis). DNA from Borrelia burgdorferi was found in brain tissue or spinal fluid in all three cases. Surprisingly, none of the patients had Lyme antibodies in their spinal fluid, showing that a negative spinal fluid test does not rule out LNB.
Two of the patients improved greatly after long-term antibiotic treatment. Their brain lesions got smaller or disappeared. One of them had new lesions throughout the course of treatment, but these too were resolved with more antibiotics.
Long-Term Antibiotic Treatment Results
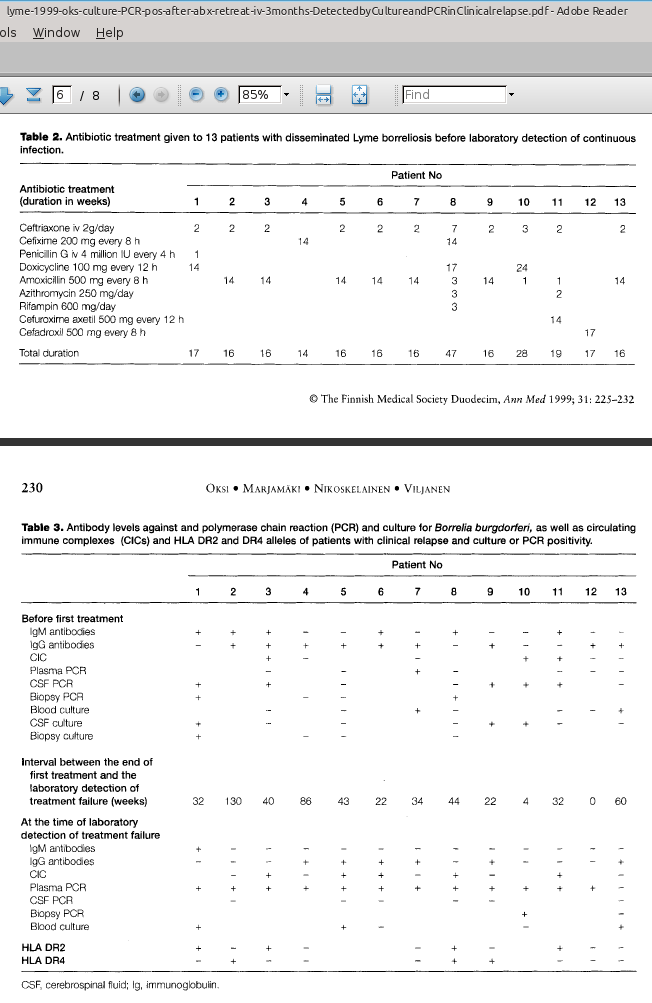
A 1998 study by Oksi et al. found that long-term antibiotic treatment led to significant recovery in 90% of patients with disseminated Lyme disease, many of whom presented with serious neurological and musculoskeletal symptoms. Patients treated with only oral cefixime (200 mg) and probenecid (500 mg) for 100 days were more likely to relapse and test positive for Borrelia DNA by a polymerase chain reaction test (PCR) months later. In contrast, those who received 2 grams of intravenous ceftriaxone for 14 days, followed by oral amoxicillin (500 mg) with probenecid (500 mg) three times daily for 100 days, had better outcomes. However, some individuals still tested positive for Borrelia DNA in their blood for many months after treatment, including one case 33 months later, suggesting that the bacteria may persist even after clinical recovery.
During antibiotic therapy, patients often experienced adverse effects such as fever, rash, or worsening symptoms, known as Herxheimer-like reactions. These were common, especially in the combination therapy group, but generally mild. They did not necessitate a stop to treatment.
The study challenges the assumption that Lyme disease is reliably curable with short-term antibiotics, suggesting instead that extended, individualized treatment may be necessary in cases of disseminated infection.
Relapse After Treatment Due to a Persistent Pathogen
Building on this, a 1999 study by the same research team documented relapses in patients who had already undergone more than 100 days of antibiotic therapy. Some experienced a return of symptoms months later, and Borrelia DNA or even live spirochetes were still detectable, suggesting that the infection may persist in a dormant state and later reactivate. Clinical improvement did not always indicate bacterial eradication, and standard antibody tests often failed to detect ongoing infection.
Symptoms often included cognitive and musculoskeletal issues, overlapping with conditions such as multiple sclerosis and fibromyalgia. More intensive treatment, typically involving intravenous antibiotics followed by oral regimens, seemed to reduce the risk of recurrence in some cases. However, some patients still experienced relapses despite extended therapy and confirmed infection. These findings highlight the uncertainty surrounding the optimal duration of treatment for late-stage Lyme disease. Individual differences in immune response and genetic makeup may influence the body’s ability to eliminate the infection. The authors recommend ongoing monitoring with PCR or culture to guide retreatment, and further research is needed to establish the most effective strategies.
The Possible Bacterial Cause Behind Alzheimer’s
A 2011 study by Judith Miklossy challenges the idea that Alzheimer’s disease, one of the leading causes of dementia, is exclusively a degenerative brain disorder. Alzheimer’s disease is marked by gradual cognitive decline and characteristic changes in the brain.
Alzheimer’s Disease Features:
Progressive brain shrinkage
Memory loss
Amyloid-beta plaque buildup (Once called cortical senile plaques)
Tau protein tangles (AKA neurofibrillary tangles)
While genetics and inflammation are known contributors, the underlying cause still remains unresolved. This uncertainty has led some researchers, including Miklossy, to explore whether chronic infection could be a missing piece in the puzzle.
Historic evidence supports this possibility. In the early 20th century, Noguchi and Moore linked Treponema pallidum, the syphilis spirochete, to dementia and amyloid buildup in general paresis. Miklossy argues that other spirochetes may produce similar effects, making Alzheimer’s a modern counterpart to this once-common infectious dementia.
Paresis Features:
Diffuse, predominantly frontotemporal cortical atrophy
Severe neuronal loss
Reactive microgliosis and astrocytosis
By examining brain tissue, she found that over 90% of Alzheimer’s patients had spirochetes present. Borrelia burgdorferi was found in 25.3% of cases and appeared 13 times more often in Alzheimer’s patients than in people without the disease.
Other spirochetes were also frequently identified, often located inside the brain’s amyloid plaques. When brain cells were exposed to spirochetes, they developed Alzheimer-like features, such as dementia.
This led Miklossy to propose a new interpretation of plaque formation. Rather than being mere waste products, amyloid deposits may be part of the body’s defensive response to persistent bacterial infection; structures that entrap and isolate the microbes over time.
Miklossy argues that Alzheimer’s meets the scientific criteria for an infectious disease, based on both Koch’s postulates (establishing whether a specific microbe causes a disease) and Hill’s guidelines (a set of standards used to determine if a statistical association is likely to be causal). Since these infections may begin years before symptoms appear, she calls for a major shift in how we understand and treat Alzheimer’s. With the right antibiotics and anti-inflammatory therapies, it may be possible to prevent or even reverse the disease in some cases.
Heart Conditions and Lyme Disease
A 2012 study by Kubánek et al. linked Borrelia burgdorferi to serious heart conditions, particularly dilated cardiomyopathy (DCM), a disease where the heart becomes enlarged and weakened. Borrelia DNA was found in 24 percent of patients with recent-onset DCM, while none of the control group tested positive. Spirochetes were also observed within heart tissue.
Among those who tested positive, 30 percent had B. burgdorferi sensu stricto, 20 percent had B. afzelii, and 40 percent had B. garinii. Parvovirus B19 and cytomegalovirus were detected at similar rates in both groups, suggesting Borrelia as the more likely cause of heart damage.
Many Borrelia-positive patients had autoimmune conditions like thyroiditis, Crohn’s disease, or psoriasis, along with antibodies targeting blood vessel linings. These patients often lacked classic Lyme symptoms or known tick exposure and sometimes tested negative for antibodies. Yet, some improved with targeted antibiotic treatment. This suggests that persistent Borrelia infection may provoke immune dysfunction and directly contribute to heart damage. However, improvement with antibiotics was modest, and few patients showed active inflammation, suggesting a chronic stage of infection rather than acute myocarditis.
These findings support the idea that ongoing infection, rather than autoimmunity alone, may drive heart damage in some patients. Persistent Borrelia burgdorferi sensu lato infection could be involved in the pathophysiology (a functional change following disease/injury) of individuals with DCM living in Lyme-endemic areas.
Methodological Trends Across Studies
The studies in this article consistently favored PCR testing to detect Borrelia DNA over standard antibody tests such as ELISA. This was especially important in chronic or late-stage cases, where immune markers may be absent or misleading. Several studies also examined tissue directly, using biopsy, culture, or imaging to identify bacterial presence or inflammation. These methods were particularly useful in patients with neurological symptoms that resembled other conditions like multiple sclerosis or Alzheimer’s.
Another recurring feature was the use of antibiotic response as part of the diagnostic process. In many cases, patients improved with long-term antibiotic treatment, and this clinical response helped confirm infection even when laboratory results were inconclusive. Together, these approaches reflect a practical strategy that relies on molecular tools, symptom tracking, and direct tissue analysis to understand persistent Lyme-related illness.
Diagnostic Complexity and a Need for Broader Testing
A draft study protocol from the Canadian Lyme Disease Research Network (CLyDRN) aims to create a more comprehensive testing strategy for Lyme disease and highlights how conventional methods may overlook persistent infections. The protocol includes tests for key inflammatory markers and immune complex detection, offering tools that go beyond the limited scope of standard antibody tests.
Conclusion
These studies suggest that chronic infections, especially from Borrelia burgdorferi, are likely an overlooked cause of neurological and degenerative diseases once thought to be purely genetic or autoimmune.
This growing body of research highlights the need for better diagnostic tools and a broader clinical perspective. Recognizing infection as a possible root cause could transform how we detect, treat, and even prevent conditions like ALS, Alzheimer’s, and cardiomyopathy.
Please support the Trozzi Team’s mission to drive solution-oriented research, raise awareness, empower the public, and foster meaningful change through dedicated grassroots efforts.






Excellent information.Thank you 🙏🇨🇦