Nanotechnological Investigations of COVID-19 “Vaccines.”
A whitepaper on the concerning composition of the "vaccines".
Anonymously authored by The Scientists’ Club
Introduction
The new COVID-Sars2 pandemic induced industries to develop new drugs that they called vaccines. The mechanism of action of these new drugs as declared by the pharmaceutical industries coupled with what is reported in the products’ data sheet was clear enough to made scientists understand that those products are not vaccines but nanotechnological drugs working as a genetic therapy.
The name “vaccine” is likely to be an escamotage used for bureaucratic reasons in order to get an urgent approval, so dribbling all the normal rules necessary for new drugs, especially for those involving novel nanotechnological mechanisms, never experienced before. All these “vaccines” are patented, and their actual content is kept secret even to the buyers, who, of course, use taxpayers’ money. So, consumers (taxpayers) have no information about what they receive in their bodies. They are kept in the dark as far as the nanotechnological processes involved are concerned, on the side effects on the body but mostly on the possible nano-bio-interactions that can happen.
The present study through direct analyses on a few “vaccines” by means of nanotechnological instrumentation gives information about their actual content.
Materials and Methods
Four “vaccines” were analyzed developed for Corona Virus disease (Comirnaty di Pfizer-BioNtech, Vaxzevria by Astrazeneca, Janssen by Johnson & Johnson), Moderna) using different instrumentation and protocols of preparation according to new nanotechnological approaches.
Optical Microscope, Dark-Field Microscope, UV absorbance and fluorescence spectroscope, Scanning Electron Microscopes, Transmission Electron Microscope, Energy Dispersive Spectroscope, X-ray Diffractometer, Nuclear Magnetic Resonance instruments were used to verify the “vaccines”’ morphologies and contents. For the high-technology measurements and the care of the investigation, all the controls were activated, and reference measurements adopted in order to obtain validated results.
Because of the brevity of the text, some measures are not reported here.
The analyses verified the morphology of the content of the samples and their chemical composition. The following images show in an objective way what the instrumentation detects.
Refrigerated samples were processed under sterile conditions, using laminar flow chamber and sterilized labware. Steps for analyses were:
Dilution in 0.9% sterile physiological saline (0.45 ml + 2 ml)
Polarity fractionation: 1.2 ml hexane + 120 ul of RD1 sample 3. Extraction of hydrophilic aqueous phase
UV absorbance and fluorescence spectroscopy scanning
Extraction and quantification of RNA in the sample
Electron and optical microscopy of aqueous phase
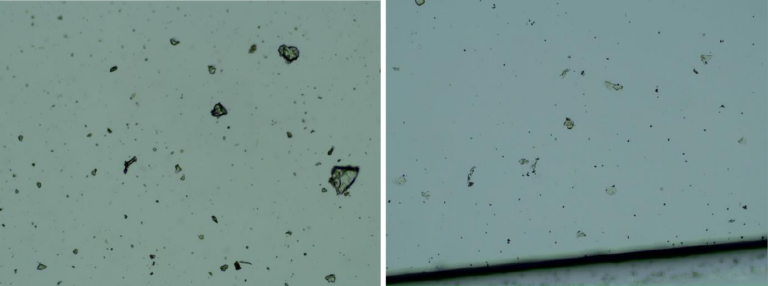
The observations under a dark-field microscope of the product by Pfizer drops revealed some entities that can be graphene strips.
Optical Microscopy
Images of the aqueous fraction of were subsequently obtained by optical to visually assess the possible presence of graphene. The observations under optical microscope of revealed abundance of transparent 2D laminar objects that show great similarity with images from literature (Xu et al, 2019), and with images obtained from rGO standard (SIGMA)(Figures 2a,b). Images of big transparent sheets of variable size and shapes were obtained, showing corrugated and flat, irregular. Smaller sheets of polygonal shapes, also similar to flakes described in literature (Xu et al, 2019) can be revealed with dark field microscopy (Fig 2c). All these laminar objects were widespread in the aqueous fraction of the sample and no component described by the registered patent can be associated with these sheets.
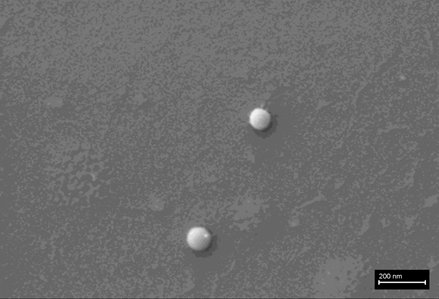
The presence of graphene in Pfizer “vaccine” is confirmed by the SEM and TEM observations.
Electronic Transmission Microscopy
In fig 2d we show TEM images of the aqueous fraction from sample, showing high similarity with TEM images of graphene oxide from the literature (Choucair et al, 2009). An intricate matrix or mesh of folded translucent flexible sheets can be observed, with a mixture of darker multilayer agglomerations and lighter colored unfolded monolayers. Darker linear areas appear due to local overlap of sheets and local arrangement of individual sheets in parallel to the electron beam. After the mesh, a high density of unidentified rounded and elliptical clear shapes appears, possibly corresponding to holes generated by mechanical forcing of the mesh during treatment.
We show here 3 images with progressive magnification:
Fig. 4a and 4b shows a TEM microscope observation where particles of graphene in a Pfizer ”vaccine” are present. The X-ray diffractometry reveals their nature of crystalline Carbon-based nanoparticles.

For a definitive identification of graphene by TEM, it is necessary to complement the observation with the structural characterization by obtaining a characteristic electron diffraction standard sample (as the figure b shown below). The standard sample corresponding to graphite or graphene has a hexagonal symmetry, and generally has several concentric hexagons.
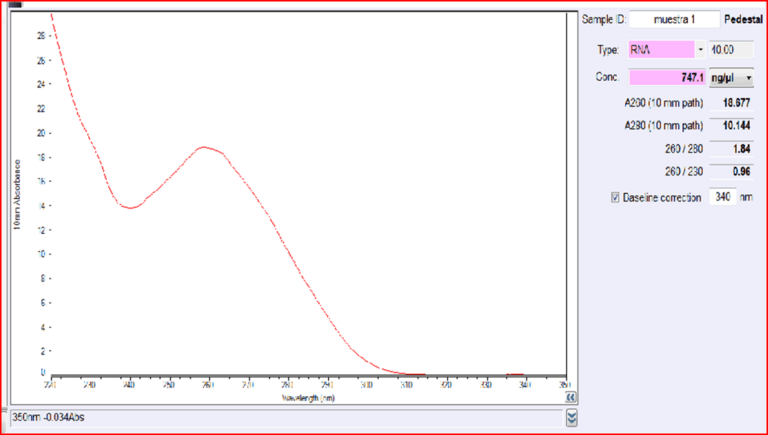
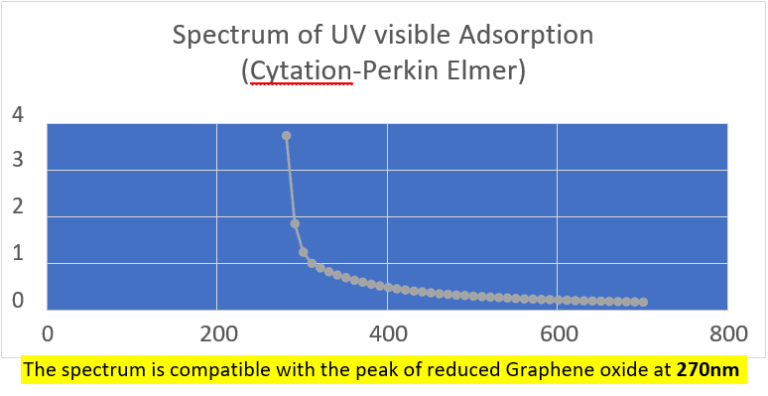
Quantification of RNA in the sample was carried out with conventional protocols (Fisher). According to NanoDropTM 2000 spectrophotometer calibration check specific software (Thermofisher), the UV absorption spectrum of total aqueous fraction was correlated to 747 ng/ul of unknown absorbing substances. However, after RNA extraction with commercial kit (Thermofisher), quantification with RNA specific Qbit fluorescence probe (Thermofisher) showed that only 6t ug/ul could be related to the presence of RNA. The spectrum was compatible with the peak of rGO at 270nm. According to microscopic images presented here, most of this absorbance might be due to graphene-like sheets, abundant in suspension in the sample. This thesis was further supported by high fluorescence from the sample with maximum at 340 nm, in accordance with peak values for GO. It must be reminded that RNA does not show spontaneous fluorescence under UV exposure.
References for the preparation 1,2,3
UV fluorescence of aqueous fraction
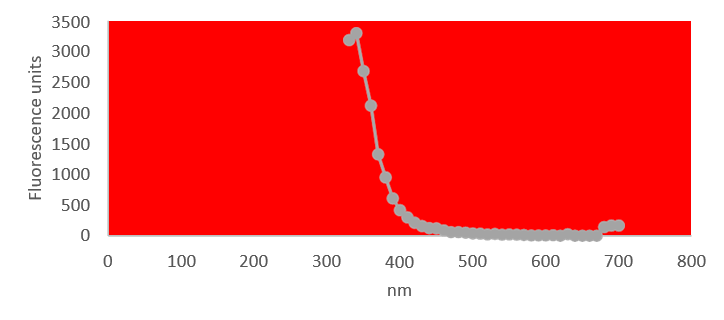
UV absorption and fluorescence spectra were obtained with Cytation 5 Cell Imaging Multi-Mode Reader Spectrophotometer (BioteK). UV absorbance spectrum confirmed a maximum peak at 270 nm, compatible with presence of rGO. UV fluorescence maximum at 340 nm also suggests presence of significant amounts of rGO in the sample (Bano et al, 2019).
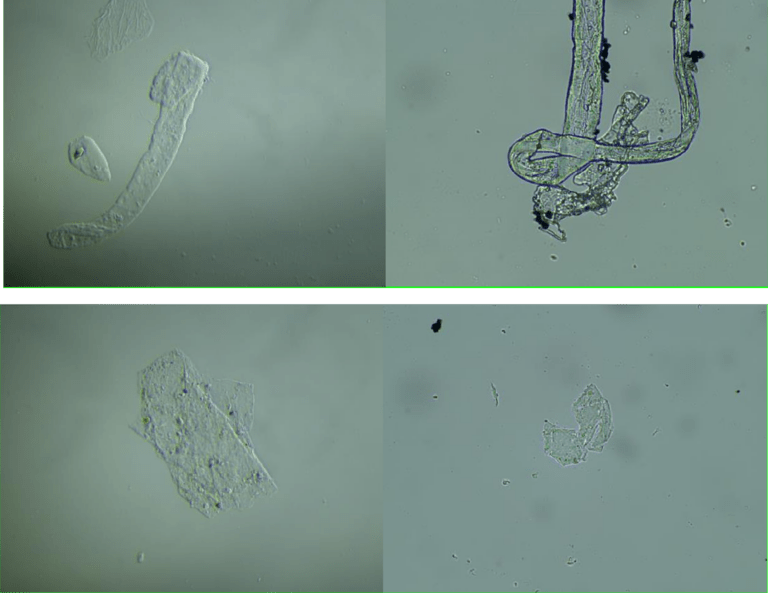
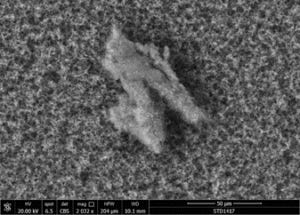
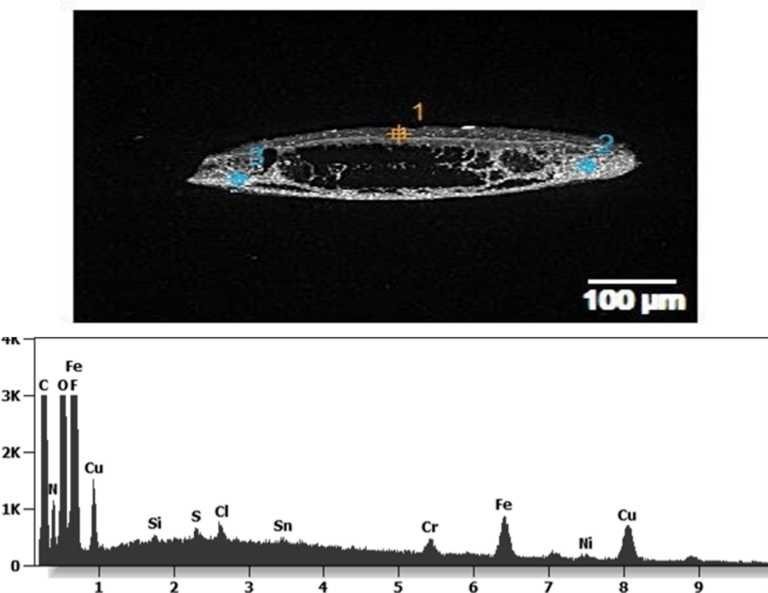
The following images show different particles identified in Pfizer, Moderna, Astrazeneca, Janssen “vaccines” analyzed under an Environmental Scanning Electron Microscope coupled with an x-ray microprobe of an Energy Dispersive System that reveals the chemical nature of the debris observed.
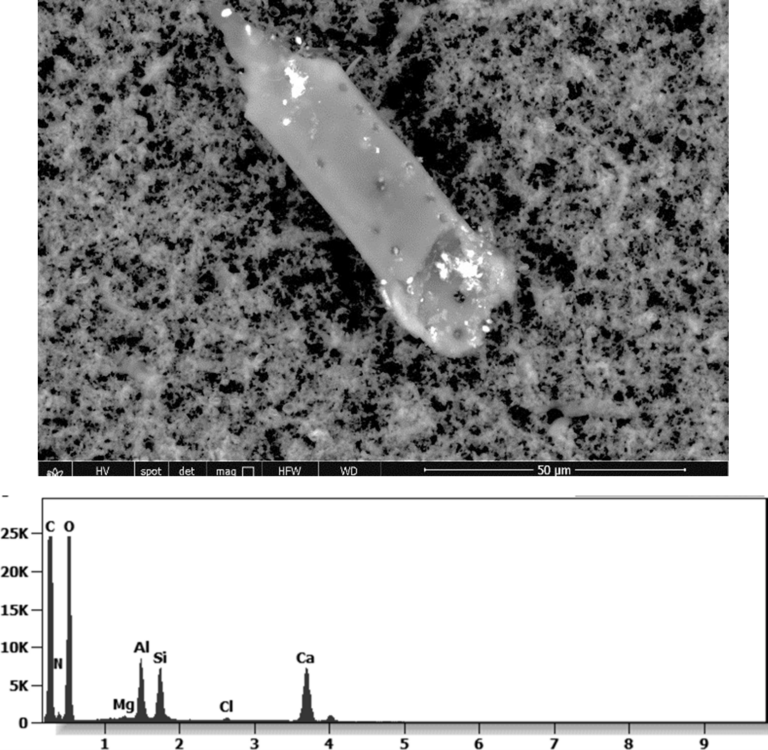
The 50-micron long body is a mysterious presence in a vaccine. It could be a trypanosomal parasite.
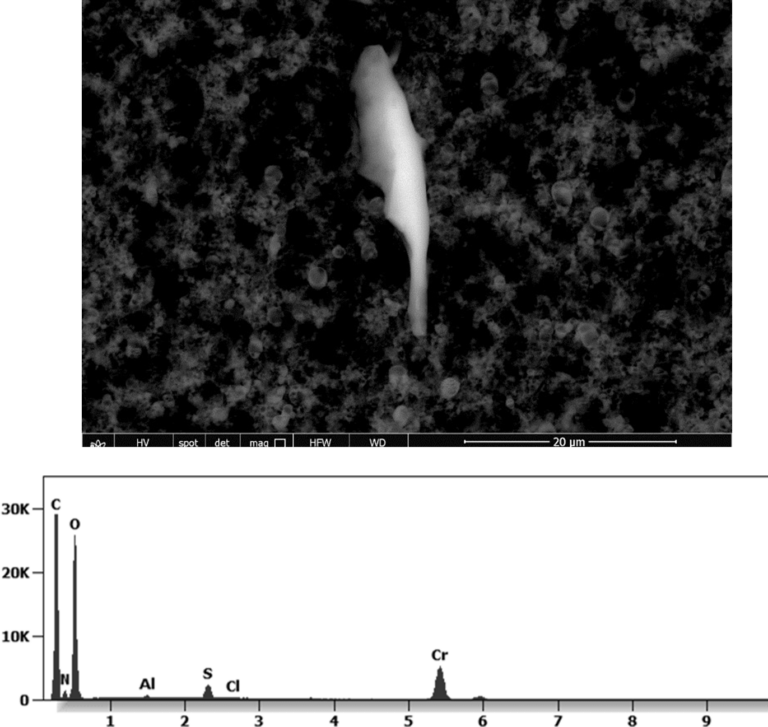
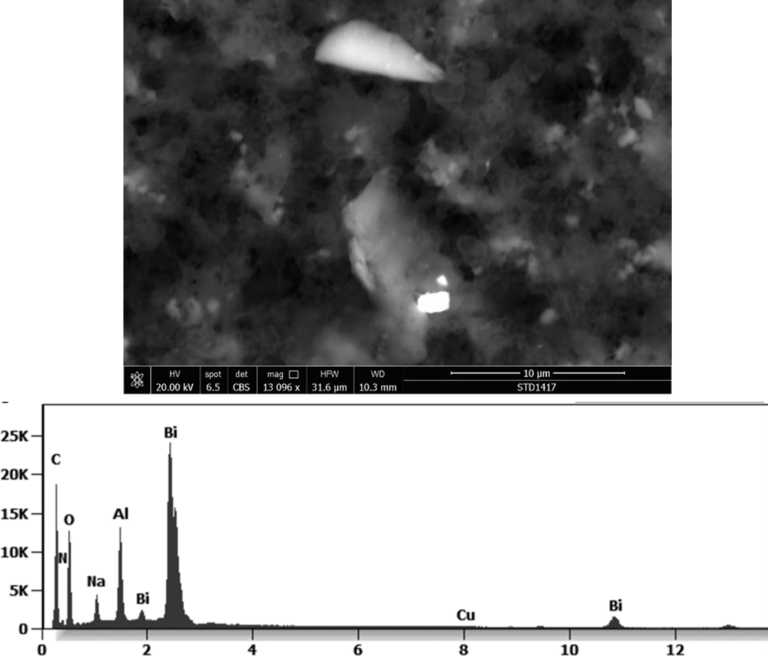
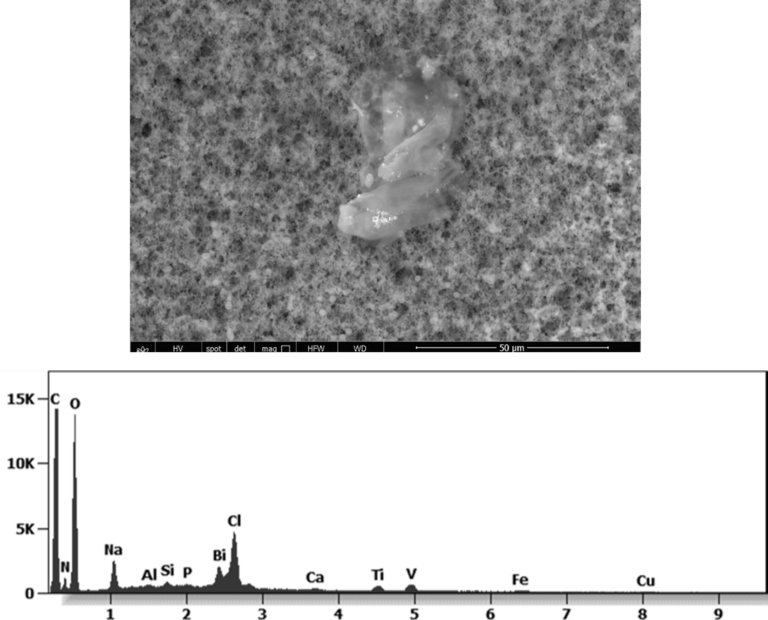
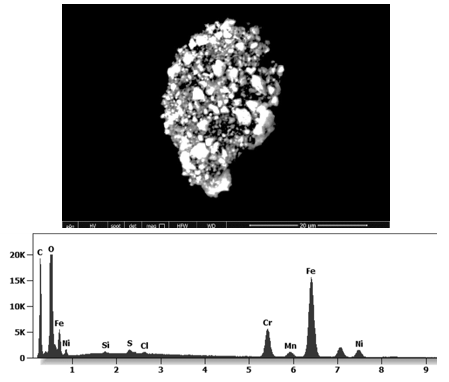
This aggregate is magnetic and can trigger biological problems inside the blood circulation due to possible interactions with other dipoles.
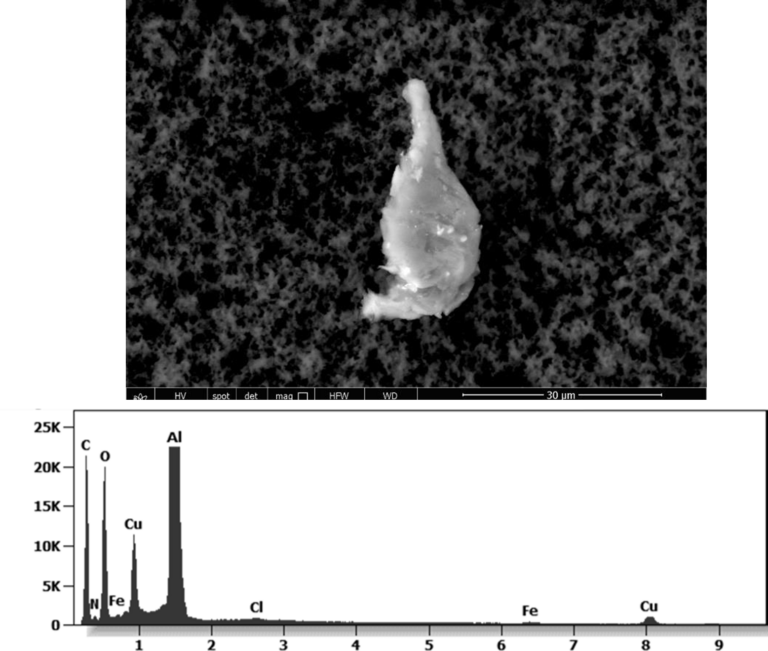
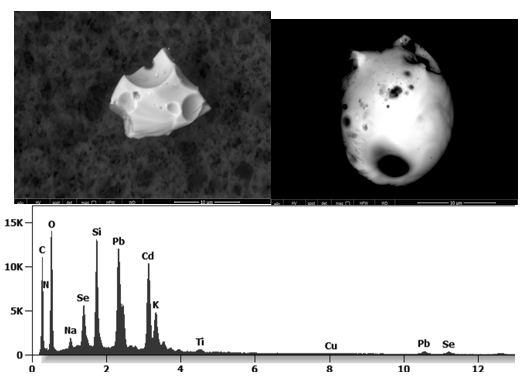

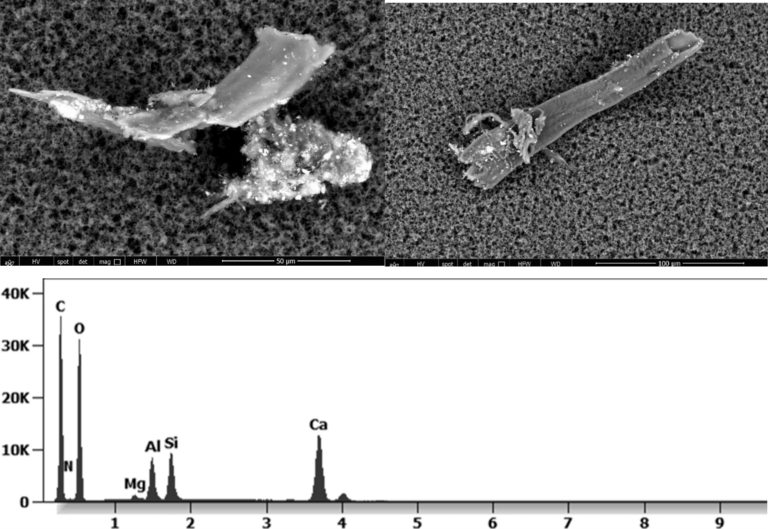
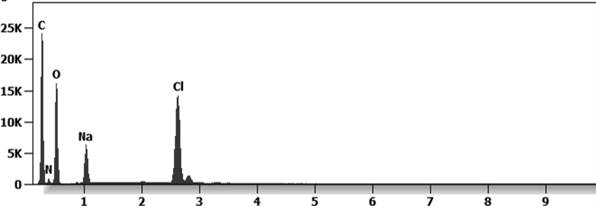
Other analyses with an XRF (X-ray fluorescence) instrument reveal the organic part of which the Astrazeneca “vaccine” is composed.
Discussion
The analyzed “vaccines” present components that are not mentioned in the technical data sheet and whose presence does not seem to have to do with the concept of vaccine. Since they are not included in the documentation presented to the Governmental organizations (FDA, EMA, etc.) for the legal approval aimed at the commercialization and the human use, they seem to be a contamination probably due to the industrial process of manufacturing. It seems that nobody controlled the final product before its distribution. That means that consumers are not informed of the real content of the products. Possible side effects may be due to the injection of those contaminants into the body. It must be observed that the components that are not declared but we
identified are not biocompatible and some have also a mechanical impact once they are inside the blood circulation, especially in contact with the vascular endothelium.
The entities present in Pfizer and Astrazeneca “vaccines”, identified by the ESEM images, can represent a risk for the human body. They can be responsible of the formation of thrombi, since they are thrombogenic. A further risk is represented by the extravasation of the particles with an ensuing possible haemorrhage. Once in the blood circulation, the particles can be carried also to the brain. In this case the patient can suffer from a stroke, and/or a cerebral haemorrhage. If the damage of the endothelium caused by the particles occurs in the heart, there is a high probability of contracting a myocarditis. In addition to all that, the toxicity of graphene is well-known.
The presence of non-biocompatible organic-inorganic foreign bodies in the blood circulation can be responsible of a nano-bio-interaction that can induce severe health problems.
References
Bano, I. et al , 2019. Exploring the fluorescence properties of reduced graphene oxide with tunable device performance, Diamond and Related Materials, Volume 94,59-64,ISSN 0925- 9635, (Read here)
Biroju, Ravi & Narayanan, Tharangattu & Vineesh, Thazhe Veettil. (2018). New advances in 2D electrochemistry—Catalysis and Sensing. 10.1201/9781315152042-7.
Choucair, M., Thordarson, P. & Stride, J. Gram-scale production of graphene based on solvothermal synthesis and sonication. Nature Nanotech 4, 30–33 (2009). (Read here)
Kim et al, Seeing graphene-based sheets, Materials Today,Volume 13, Issue 3,2010, Pages 28- 38,ISSN 1369-7021, (Click here)
Xu et al, (2019) Identification of graphene oxide and its structural features in solvents by optical microscopy, RSC Adv., 9, 18559-18564
Extracction RNA Kit (Click here)
NanoDrop™ (Click here)